Basic Atomic Structure⁚ A Comprehensive Overview
This section provides a concise overview of fundamental atomic concepts․ We will explore the building blocks of matter and their arrangement, laying the groundwork for a deeper understanding of chemistry․
Atoms are the fundamental building blocks of all matter․ Everything around us, from the air we breathe to the ground we walk on, is composed of these incredibly tiny particles․ While invisible to the naked eye, atoms possess a rich internal structure that governs their properties and interactions․ Understanding atoms is crucial for comprehending the behavior of matter at both macroscopic and microscopic levels․ The concept of the atom has evolved significantly throughout history, from ancient philosophical speculations to the sophisticated models we utilize today․ Early thinkers conceived of indivisible particles, but modern science reveals a much more intricate reality․ Atoms are not, in fact, indivisible; they are composed of even smaller subatomic particles, each with its unique characteristics․ This exploration delves into the fascinating world of atomic structure, providing a foundation for understanding the intricacies of matter and the physical world․ Exploring the atom’s components and their arrangement will unlock a deeper appreciation for the fundamental principles of chemistry and physics․ Further investigation into atomic behavior will reveal how these minuscule entities govern the macroscopic properties of materials․ The journey into the atomic realm unveils the intricate dance of subatomic particles and their collective interactions, shaping the world we inhabit․
Subatomic Particles⁚ Protons, Neutrons, and Electrons
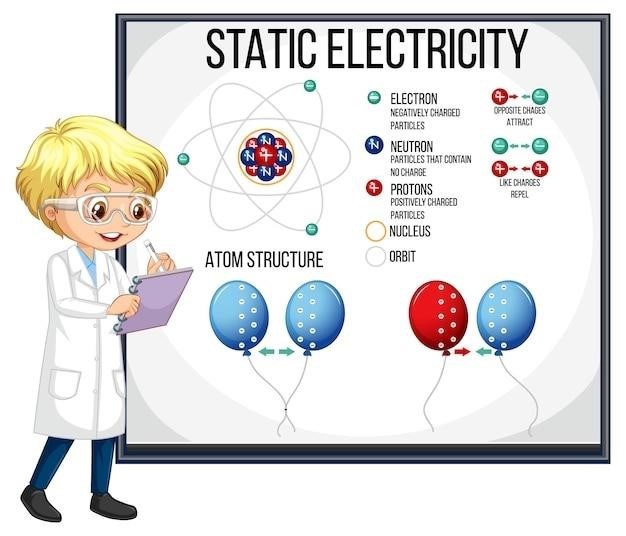
Atoms are not indivisible; they consist of three primary subatomic particles⁚ protons, neutrons, and electrons․ Protons carry a positive electrical charge (+1), while electrons carry a negative charge (-1)․ Neutrons, as their name suggests, are electrically neutral․ Protons and neutrons reside within the atom’s nucleus, a dense central region, while electrons orbit the nucleus at varying distances․ The mass of a proton is approximately equal to the mass of a neutron, significantly larger than the mass of an electron․ This mass difference is crucial in understanding atomic properties and behavior․ The number of protons in an atom’s nucleus defines its atomic number, which uniquely identifies an element on the periodic table․ The positive charge of the protons is balanced by the negative charge of the electrons, resulting in an electrically neutral atom in its ground state․ However, atoms can gain or lose electrons, leading to the formation of ions, which carry a net electrical charge․ The arrangement of electrons in energy levels surrounding the nucleus determines the atom’s chemical behavior and its ability to form bonds with other atoms․ Understanding the properties and interactions of these subatomic particles is fundamental to grasping the nature of matter and the forces that govern it․ The relative masses and charges of these particles dictate the chemical and physical properties of elements and compounds․
The Nucleus⁚ Heart of the Atom
At the center of every atom lies the nucleus, a tiny, incredibly dense region containing most of the atom’s mass․ This nucleus is composed of two types of subatomic particles⁚ protons and neutrons, collectively known as nucleons․ The protons, each carrying a positive charge, determine the atom’s atomic number and thus its identity as a specific element․ The neutrons, possessing no electrical charge, contribute to the atom’s mass but not its charge․ The strong nuclear force, a powerful fundamental force, overcomes the electrostatic repulsion between the positively charged protons, holding the nucleus together․ The stability of the nucleus is crucial for the stability of the atom as a whole․ A nucleus with an unstable ratio of protons to neutrons can undergo radioactive decay, emitting particles or energy to achieve a more stable configuration․ The size of the nucleus is remarkably small compared to the overall size of the atom; if an atom were the size of a football stadium, the nucleus would be only about the size of a pea at its center․ This vast majority of the atom’s volume is occupied by the electrons orbiting the nucleus, which contribute minimally to the overall atomic mass․ The structure and stability of the nucleus are critical in determining an element’s properties and its behavior in chemical reactions․
Electron Shells and Energy Levels
Electrons, negatively charged subatomic particles, occupy regions of space surrounding the nucleus called electron shells or energy levels․ These shells are not physical structures but rather represent regions where electrons are most likely to be found․ Each shell can hold a specific maximum number of electrons; the first shell closest to the nucleus holds a maximum of two electrons, while subsequent shells can accommodate more․ The electrons in the outermost shell are called valence electrons and are crucial in determining an atom’s chemical behavior and reactivity․ Electrons within a shell possess specific energy levels; those in shells farther from the nucleus have higher energy levels․ Electrons can transition between energy levels by absorbing or emitting energy in the form of photons (light)․ Absorption of energy causes an electron to jump to a higher energy level (excited state), while emission of energy results in the electron falling back to a lower energy level (ground state)․ This energy transition is often accompanied by the emission or absorption of light of specific wavelengths, forming the basis of atomic spectroscopy, a technique used to identify elements․ The arrangement of electrons in shells and their energy levels dictates an atom’s chemical properties and its ability to form bonds with other atoms․
Atomic Number and Mass Number
Two key numbers characterize each element⁚ the atomic number and the mass number․ The atomic number (Z) represents the number of protons in an atom’s nucleus․ This number is unique to each element and defines its identity; all atoms of a given element have the same atomic number․ For example, all carbon atoms have an atomic number of 6, meaning they each contain six protons․ The periodic table arranges elements in order of increasing atomic number․ The mass number (A), on the other hand, represents the total number of protons and neutrons in an atom’s nucleus․ Unlike the atomic number, the mass number can vary for atoms of the same element, giving rise to isotopes․ To calculate the number of neutrons in an atom, subtract the atomic number (number of protons) from the mass number (total number of protons and neutrons)․ For instance, a carbon-12 atom (12C) has a mass number of 12 and an atomic number of 6, indicating it possesses 6 protons and 6 neutrons (12 ― 6 = 6)․ Understanding atomic number and mass number is fundamental to comprehending the structure and properties of elements and their isotopes, crucial for various applications in chemistry and nuclear physics․
Isotopes⁚ Variations in Atomic Mass
Isotopes are atoms of the same element that share the same atomic number (number of protons) but differ in their mass number (total number of protons and neutrons)․ This variation arises from a differing number of neutrons in the atom’s nucleus․ While the number of protons determines the element’s identity, the number of neutrons can fluctuate without altering the element’s fundamental chemical properties․ For example, carbon-12 (12C) and carbon-14 (14C) are isotopes of carbon․ Both have six protons (atomic number 6), but 12C has six neutrons, while 14C has eight neutrons․ This difference in neutron count leads to a variation in their atomic mass․ Many elements exist as mixtures of isotopes in nature․ The relative abundance of each isotope influences the element’s average atomic mass, which is a weighted average reflecting the proportion of each isotope present․ Isotopes have various applications, including radiocarbon dating (using 14C) to determine the age of organic materials, and in medical imaging and treatment using radioactive isotopes․ Understanding isotopes is crucial for comprehending nuclear reactions and the behavior of elements in various contexts․ The differences in mass between isotopes, while subtle at the atomic level, can have significant macroscopic implications․
Ions⁚ Charged Atoms
Ions are atoms or molecules that carry a net electrical charge․ This charge arises from an imbalance in the number of protons (positively charged) and electrons (negatively charged) within the atom or molecule․ When an atom gains one or more electrons, it acquires a negative charge and becomes an anion․ Conversely, when an atom loses one or more electrons, it develops a positive charge and becomes a cation․ The formation of ions is a fundamental process in chemical bonding and reactions․ Ionic compounds are formed through the electrostatic attraction between oppositely charged ions․ For instance, sodium (Na) readily loses one electron to form a sodium cation (Na+), while chlorine (Cl) readily gains one electron to form a chloride anion (Cl–)․ The electrostatic attraction between Na+ and Cl– leads to the formation of sodium chloride (NaCl), common table salt․ The magnitude of the charge on an ion is indicated by a superscript number following the element symbol․ For example, a magnesium ion with a 2+ charge is represented as Mg2+, indicating the loss of two electrons․ The charge on an ion significantly influences its chemical behavior and reactivity․ Ions play crucial roles in various biological processes, such as nerve impulse transmission and muscle contraction, where the movement of ions across cell membranes generates electrical signals․ Understanding ionic charges is essential for interpreting chemical reactions and predicting the properties of ionic compounds․
Representing Atoms⁚ Atomic Notation
A concise and standardized method for representing atoms is crucial in chemistry․ This is achieved through atomic notation, a symbolic representation that encapsulates key information about an atom’s composition․ The notation typically uses the element symbol, along with superscripts and subscripts to denote the number of protons, neutrons, and electrons․ The element symbol, a one or two-letter abbreviation (e․g․, H for hydrogen, O for oxygen), identifies the type of atom․ A superscript to the upper left of the symbol represents the mass number (A), which is the total number of protons and neutrons in the atom’s nucleus․ A subscript to the lower left of the symbol indicates the atomic number (Z), representing the number of protons․ For example, 126C represents a carbon atom with a mass number of 12 and an atomic number of 6․ This implies that the carbon atom contains 6 protons and (12-6=6) 6 neutrons․ Since atoms are electrically neutral, the number of electrons equals the number of protons (6 in this case)․ This notation is particularly useful when dealing with isotopes, which are atoms of the same element with varying numbers of neutrons․ Different isotopes of an element will have the same atomic number but different mass numbers․ Atomic notation provides a clear and efficient way to distinguish between different isotopes and understand their nuclear composition․ Mastering atomic notation is fundamental for comprehending chemical formulas and equations․ The ability to interpret and utilize atomic notation is a cornerstone of chemical literacy․
Worksheet Examples and Solutions
Let’s solidify our understanding with some practical examples․ Consider a worksheet problem asking to determine the number of protons, neutrons, and electrons in an atom of 168O (oxygen-16)․ Using atomic notation, we can readily extract this information․ The subscript 8 represents the atomic number (Z), indicating 8 protons․ The superscript 16 is the mass number (A), representing the sum of protons and neutrons․ Since there are 8 protons, the number of neutrons is 16 ― 8 = 8․ As atoms are electrically neutral, the number of electrons equals the number of protons, which is 8․ Another example might involve identifying an element given its atomic number and mass number․ If a problem states an atom has 11 protons and 12 neutrons, we first find the atomic number (11), which corresponds to sodium (Na) on the periodic table․ The mass number (A) is the sum of protons and neutrons (11 + 12 = 23)․ Therefore, the atomic notation for this atom is 2311Na․ Finally, a question could require you to write the atomic notation given the number of each subatomic particle; If an atom has 17 protons, 18 neutrons, and 17 electrons, the atomic number is 17 (chlorine, Cl), and the mass number is 17 + 18 = 35․ The atomic notation is thus 3517Cl․ These examples illustrate the practical application of atomic notation in solving problems related to atomic structure․
Applying Atomic Structure Concepts
Understanding basic atomic structure is crucial for comprehending various chemical phenomena․ For instance, the concept of atomic number directly relates to an element’s chemical properties and its position on the periodic table․ Elements within the same group (vertical column) exhibit similar chemical behaviors due to having the same number of valence electrons—electrons in the outermost shell․ These valence electrons participate in chemical bonding, dictating how atoms interact to form molecules and compounds․ The concept of isotopes, atoms of the same element with differing neutron numbers, explains variations in atomic mass observed in nature․ Isotopes have identical chemical properties but slightly different physical properties due to their varying mass․ This understanding is vital in fields like nuclear chemistry and radioactive dating․ Furthermore, knowledge of electron shells and energy levels helps explain the behavior of atoms in chemical reactions․ Electrons can transition between energy levels, absorbing or releasing energy in the process․ This energy transfer is fundamental to spectroscopy and understanding the interactions of light and matter․ Finally, the formation of ions, charged atoms resulting from electron gain or loss, directly affects chemical bonding and the properties of ionic compounds․ Therefore, grasping fundamental atomic structure lays the groundwork for comprehending more complex chemical concepts and applications․
Further Exploration of Atomic Theory
Beyond the basics, delve into the fascinating complexities of atomic theory․ Explore the quantum mechanical model of the atom, which replaces the simplistic Bohr model with a more accurate, albeit more complex, description of electron behavior․ Understand how orbitals, regions of space where electrons are most likely to be found, are described by quantum numbers, and how these numbers dictate the electron’s energy and spatial distribution․ Investigate the principles of quantum mechanics, including wave-particle duality and the Heisenberg uncertainty principle, which fundamentally alter our understanding of the atom’s structure․ Consider the implications of electron configurations and how these configurations relate to an element’s chemical reactivity and position on the periodic table․ Explore the concepts of atomic size, ionization energy, and electronegativity, and how these properties vary across the periodic table, influencing chemical bonding and reactivity․ Investigate the advanced concepts of electron spin, magnetic moments, and the Pauli exclusion principle, which further refine our understanding of electron arrangement within the atom․ Finally, research the historical development of atomic theory, from Dalton’s atomic model to the current quantum mechanical model, appreciating the contributions of scientists like Thomson, Rutherford, and Bohr along the way․ By exploring these advanced topics, you will gain a deeper and more nuanced understanding of atomic structure and its implications in chemistry and related fields․
